All are silicates but not all are Silicoglycidol
What are silicates, structure and functions
Silicates, a union of silicon and oxygen, also called silicic acid salts, are the most abundant minerals in the earth’s crust. They occupy 92% of the continental surface, constituting basic components of rocks, sands and clays.
There are many different types of silicates. They all have in common that they are polymers of a basic structural unit, which is the silicon tetrahedron (SiO4), with a silica atom in the center, and four oxygen atoms at the vertices. These basic functional structures can be joined together to form large polymers with an indeterminate number of them.
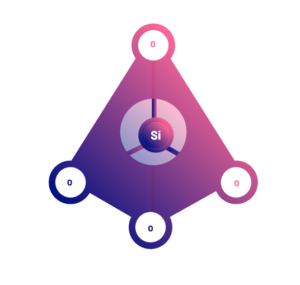
However, the bonds to each other are different, and the polymers take on different three-dimensional structures. In this way, the different structures are what determine the different types of silicates.
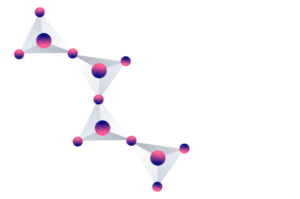
It is common for silicates to contain atoms of some other element in their composition, such as aluminum, sodium or calcium.



How to differentiate the silicates?
It is important to indicate that their properties and functions will depend on the structure of the silicates. In this sense, there are silicates that, like some bentonites, may have properties in binding to other molecules, while other bentonites do not have these properties.
To differentiate between silicates, therefore, it is necessary to recognize their structure. This is done by X-ray diffraction or electronic pictography, in which, using special devices, the three-dimensional arrangement of its atoms is obtained, identifying those silicates with mycotoxin adsorbing properties, such as Alquerfeed Antitox, and other silicates without this functionality.

